Intramolecular α-Oxygenation of Amines via N-Heterocyclic Carbene-Catalyzed Domino Reaction of Aryl Aldehyde: Experiment and DFT Calculation | CCS Chem
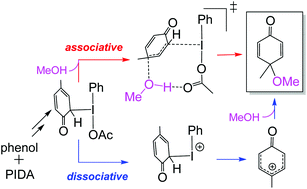
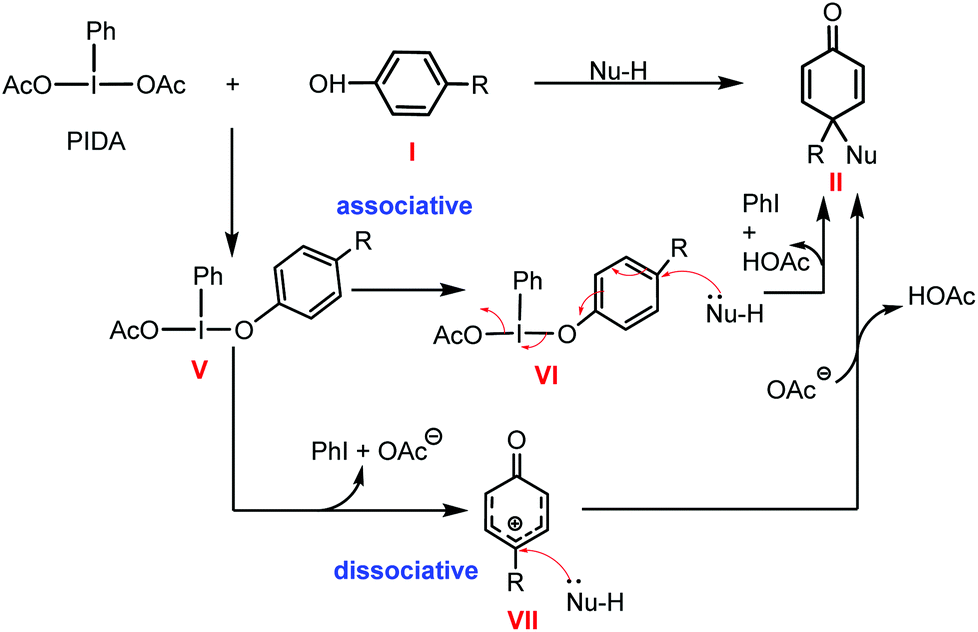
DFT mechanistic investigation into phenol dearomatization mediated by an iodine(iii) reagent - Organic & Biomolecular Chemistry (RSC Publishing)

Proposed mechanism for the oxidation of ortho substituted phenols by... | Download Scientific Diagram

Asymmetric oxidative dearomatizations promoted by hypervalent iodine(III) reagents: an opportunity for rational catalyst design? - ScienceDirect
![Molecules | Free Full-Text | Enhanced Reactivity of [Hydroxy(tosyloxy)iodo]benzene in Fluoroalcohol Media. Efficient Direct Synthesis of Thienyl(aryl)iodonium Salts | HTML Molecules | Free Full-Text | Enhanced Reactivity of [Hydroxy(tosyloxy)iodo]benzene in Fluoroalcohol Media. Efficient Direct Synthesis of Thienyl(aryl)iodonium Salts | HTML](https://www.mdpi.com/molecules/molecules-15-01918/article_deploy/html/images/molecules-15-01918-g004.png)
Molecules | Free Full-Text | Enhanced Reactivity of [Hydroxy(tosyloxy)iodo]benzene in Fluoroalcohol Media. Efficient Direct Synthesis of Thienyl(aryl)iodonium Salts | HTML

Asymmetric oxidative dearomatizations promoted by hypervalent iodine(III) reagents: an opportunity for rational catalyst design? - ScienceDirect

Synthesis of α-sulfonyloxyketones via iodobenzene diacetate (PIDA)-mediated oxysulfonyloxylation of alkynes with sulfonic acids - RSC Advances (RSC Publishing) DOI:10.1039/C7RA11875A

Intramolecular α-Oxygenation of Amines via N-Heterocyclic Carbene-Catalyzed Domino Reaction of Aryl Aldehyde: Experiment and DFT Calculation | CCS Chem

organic chemistry - Mechanism of oxidative dearomatisation with hypervalent iodine - Chemistry Stack Exchange
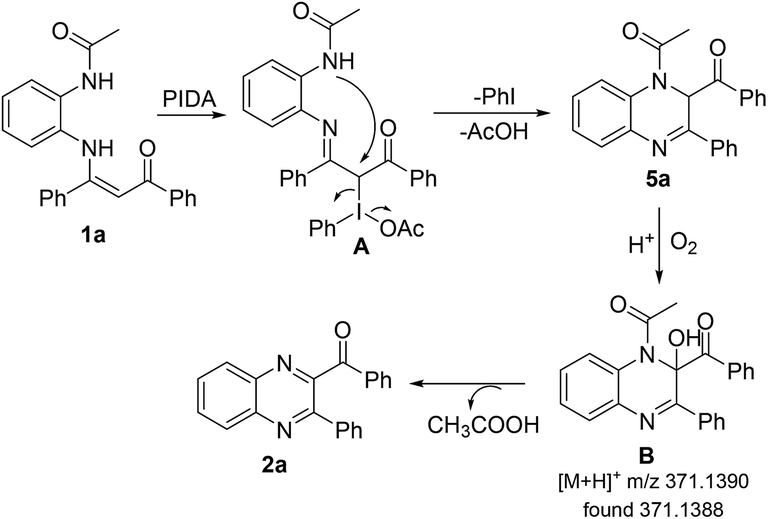
PIDA-mediated intramolecular oxidative C–N bond formation for the direct synthesis of quinoxalines from enaminones - RSC Advances (RSC Publishing) DOI:10.1039/C9RA01200A
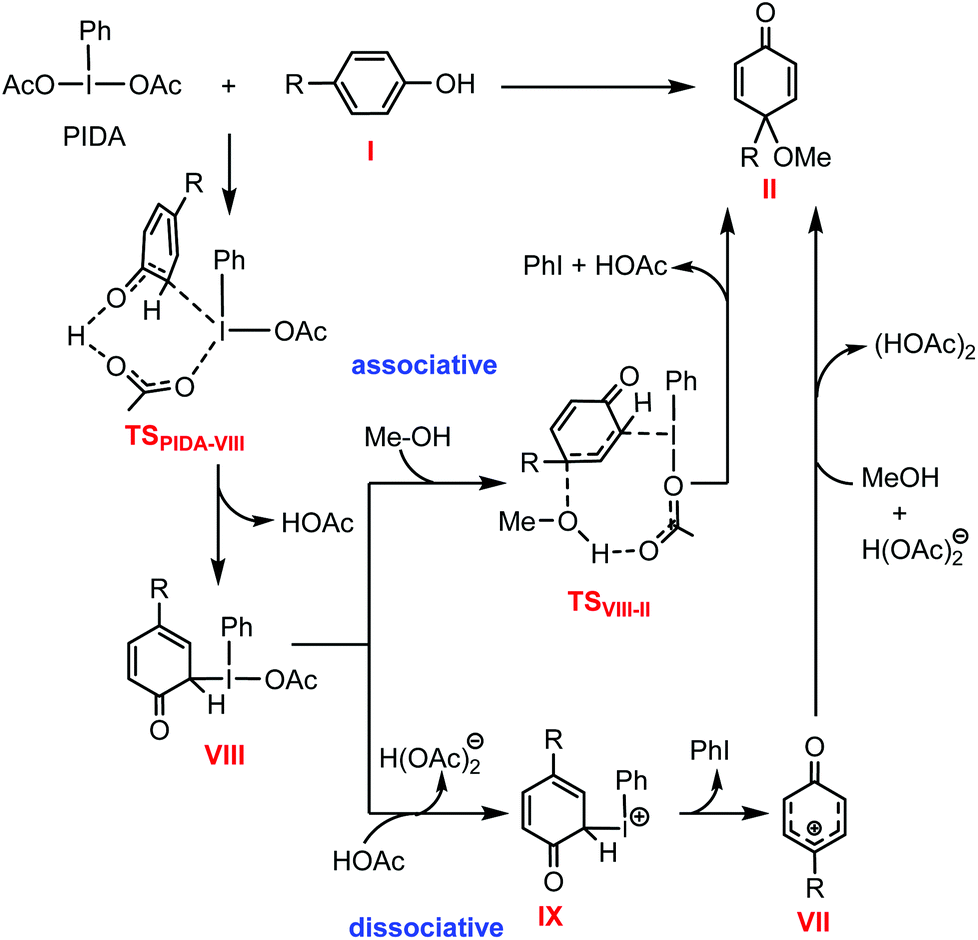
Mechanistic investigation into phenol oxidation by IBX elucidated by DFT calculations - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C9OB02650A
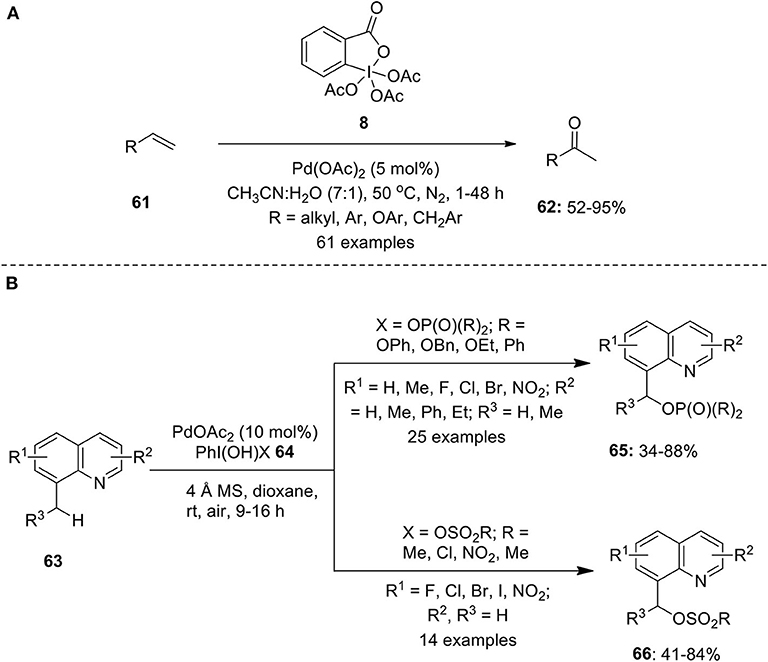
Frontiers | Hypervalent Iodine Reagents in Palladium-Catalyzed Oxidative Cross-Coupling Reactions | Chemistry

Pioneering Metal‐Free Oxidative Coupling Strategy of Aromatic Compounds Using Hypervalent Iodine Reagents - Kita - 2015 - The Chemical Record - Wiley Online Library